Introduction: Veno-occlusive disease/sinusoidal obstructive syndrome (VOD/SOS) is a potentially life-threatening complication after hematopoietic cell transplant (HCT). Defibrotide (DF), a mixture of single and double stranded oligonucleotides with profibrinolytic, anti-thrombotic, anti-ischemic and anti-inflammatory activity was approved by the FDA for the treatment of VOD/SOS in 2016. Prior to its FDA approval, its use was mostly restricted to patients with moderate to severe VOD characterized by multiorgan dysfunction. However, following approval, it has been used more liberally. We herein report a "real world experience" for commercially available DF in patients who developed VOD/SOS at our transplant center.
Methods: All patients who received DF for the treatment of VOD/SOS after allogeneic HCT from March 2016 until June 2019 were included in this study. Baseline demographic data were retrieved from the Dana Farber Cancer Institute BMT repository, and chart review was performed to obtain additional information for cases. All received DF 6.25 mg/kg/dose every 6 hours for 21-day cycle for the treatment of VOD/SOS. Patients were diagnosed with VOD/SOS if they met at least 2 of the EBMT criteria; total bilirubin ≥ 2mg/dL, painful hepatomegaly, ascites, weight gain >5% or ultrasonographic evidence of VOD. VOD/SOS severity grading was defined per the EBMT VOD guidelines.
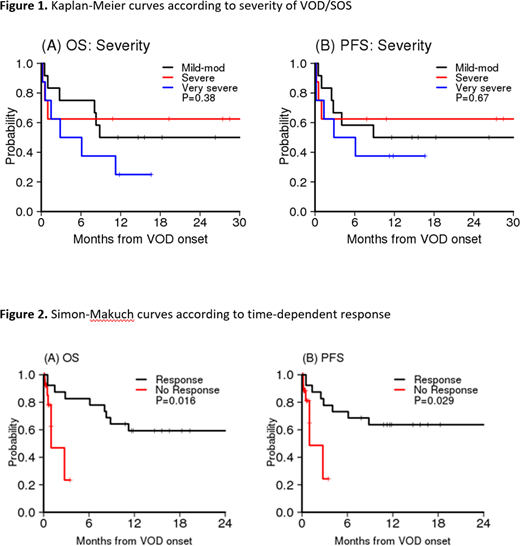
Results: Twenty-eight patients received DF for the management of VOD/SOS. Of these, 12, 8, and 8 patients had mild-moderate, severe, and very severe VOD/SOS respectively. Median time to diagnosis of VOD/SOS was day +25 after HCT. Five cases were confirmed by liver biopsy and 16 patients had reversal of flow on ultrasound. Twelve and 16 patients received myeloablative and non-myeloablative regimens respectively. DF was started on the day of diagnosis in 71% of patients. Four patients had received inotuzumab ozogamicin prior to VOD/SOS diagnosis. Twenty-one (75%) patients had resolution of VOD/ SOS, defined by complete resolution of clinical symptoms and improvement of laboratory data ascertained via detailed chart review, at a median of 14 days from starting DF. Patients with very severe VOD/SOS took the longest to respond at a median of 21 days as compared to 10 and 11.5 days in the mild-moderate and severe group, respectively (P=0.04). Survival at 28 and 56 days from VOD/SOS diagnosis was 82% and 71% respectively. In univariable analysis, the presence of pulmonary dysfunction (PD) at VOD/SOS diagnosis and early in the course of VOD/SOS was significantly associated with lack of response. Only 1 out of 7 patients with PD responded (14%) as opposed to 20 out of 21 (95%) patients without PD who responded (p=0.0001). With the median follow up of 18 months from the VOD/SOS onset (range 11,33), 1-year overall survival (OS) was 46% for the entire cohort. Disease severity was not associated with OS (p=0.38) or progression-free survival (PFS) (p=0.67) (Figure 1), but response to DF (treated as a time dependent variable in Cox model) was significantly associated with OS (HR 0.14, 95% CI 0.03, 0.69, P=0.016) and PFS (HR 0.19, 95% CI 0.04, 0.84, P=0.029) (Figure 2). All 7 non-responders died within 3 months of onset. Immediate causes of death in non-responders were VOD/SOS (n=2), grade III/IV gastrointestinal hemorrhage (n=1), relapsed disease (n=1) and sepsis (n=3). Attributed causes of death in responders were grade III/IV gut/liver graft versus host disease (n=2), relapsed disease (n=2), sepsis (n=2) and respiratory failure (n=1). Possible therapy-related adverse events were uncommon but included grade III/IV gastrointestinal hemorrhage (n=1), grade III/IV pulmonary hemorrhage (n=1), gross hematuria (n=6) and grade 3 hypotension (n=2), and proved generally manageable. Importantly, 1 out of 4 patients who received inotuzumab ozogamicin did not respond to DF and subsequently died from sepsis, considered unrelated to DF therapy.
Conclusion: We report a real world experience of DF post-FDA approval in the USA from 2016 -2019. The majority of our patients received DF as soon as VOD/SOS was diagnosed. We found that use of DF was associated with complete resolution of VOD/SOS in 75% of all patients meeting the EBMT criteria treated with DF. Our overall survival outcomes are encouraging; particularly among responders without severe adverse events. Our results favor early identification and initiation of DF in VOD/SOS after HCT.
Richardson:Celgene/BMS, Oncopeptides, Takeda, Karyopharm: Research Funding. Soiffer:Gilead: Consultancy; Juno: Other; Celgene: Other; Kiadis: Membership on an entity's Board of Directors or advisory committees; Be The Match/National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Rheos Therapeutics: Consultancy; Cugene: Consultancy; Precision Bioscience: Consultancy; Mana Therapeutics: Consultancy; VOR Biopharma: Consultancy; Alexion: Consultancy; Novartis: Consultancy. Cutler:Jazz: Consultancy, Membership on an entity's Board of Directors or advisory committees; Medsenic: Consultancy, Membership on an entity's Board of Directors or advisory committees; Generon: Consultancy, Membership on an entity's Board of Directors or advisory committees; Mesoblast: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kadmon: Consultancy, Membership on an entity's Board of Directors or advisory committees. Nikiforow:Kite: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees. Koreth:Biolojic Design Inc: Consultancy; Regeneron: Other: Research Support; Cugene: Membership on an entity's Board of Directors or advisory committees; Therakos: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: Research Support; BMS: Other: Research Support; Amgen: Consultancy; Equillium: Consultancy; Moderna Therapeutics: Consultancy; EMD Serono: Consultancy; Clinigen: Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal